Study Management System¶
Introduction
The Study Management module in Active Steward provides a solution to maintain efficient project management of studies. Study records in Active Steward are used throughout the lifecycle of a study. There are various tools which aid the use of these records, such as;
- Costs & Invoicing - Detailed cost breakdowns, managing Consortium fees and cost sharing between companies.
- Invoicing tracking, invoice generation and payment status.
- Document storage and folder structure setup specifically for study related files. Generating Meeting Documents.
- Study planning, schedules, timelines and project status tracking.
- Storage of Laboratory /CRO details.
- Track test substances.
Studies can be connected to other records in Active Steward (Constituents, Products, Entities etc.). Some study information can be presented on campaign pages for external customers to view (How to Create a Study Page in Campaigns).
Follow the guide below for a walk through of everything study related and find links to more specific tutorials.
List All Studies¶
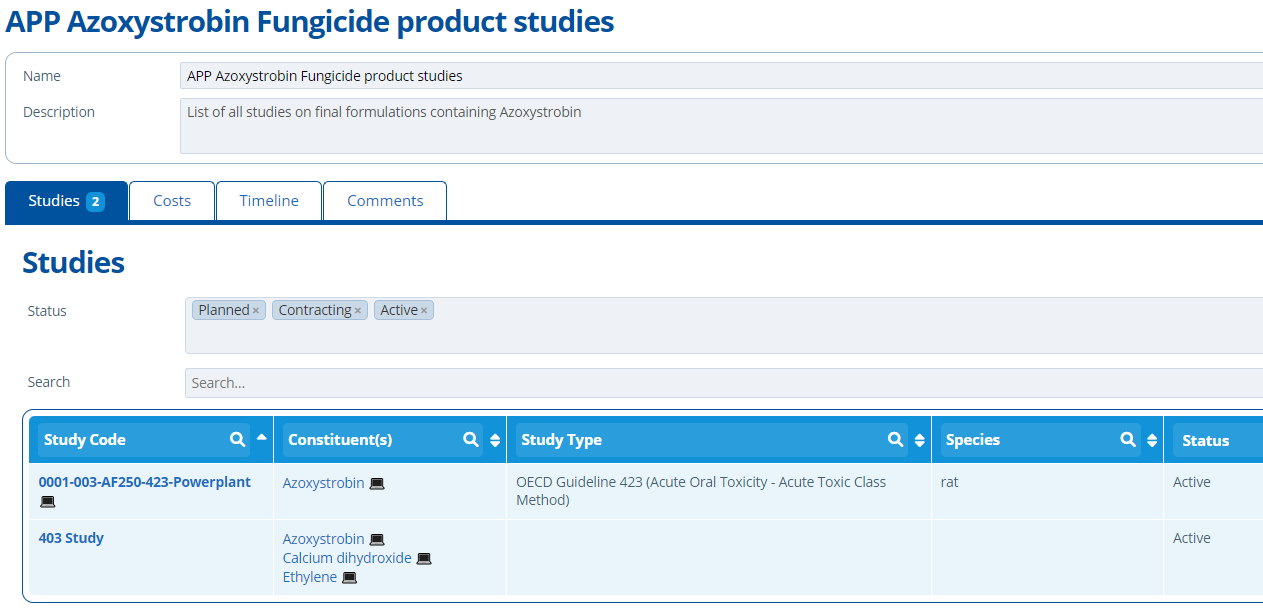
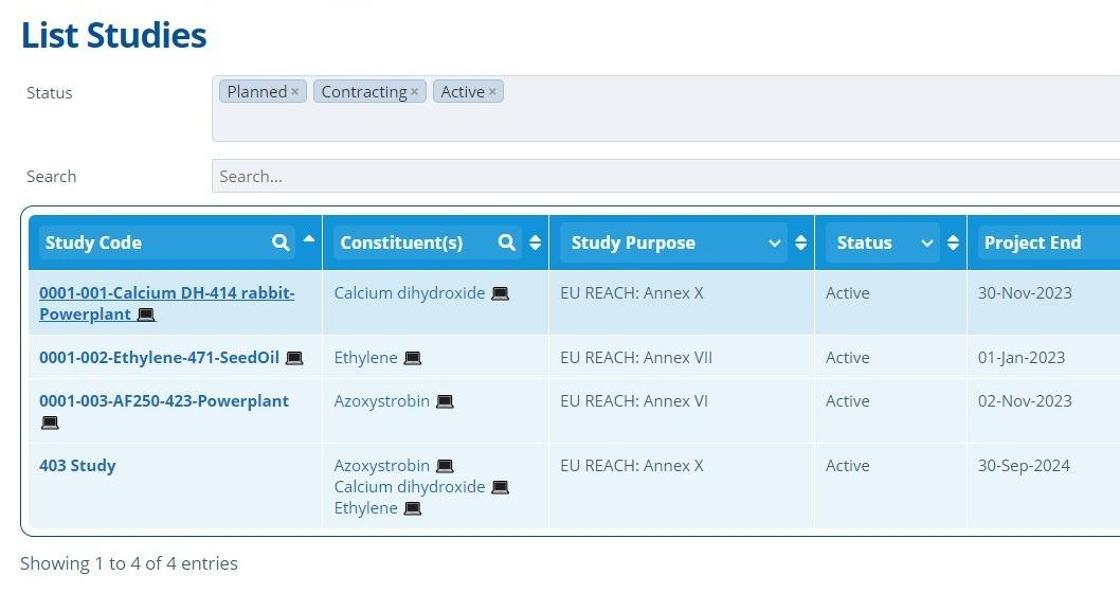
Study records are listed in the "List Studies" screen which can be found by going to "Studies" in the top navigation bar.

This page can be used to filter through all studies in the current partition. Select the study status and/or use the column headers to search. Each study project is given a unique identifying code - How to Create a Study.
To export / import study information for the whole partition, see the administration guide.
The Study Record¶
A study record is composed of multiple tabs, each with a different function explained in the below sections:

Overview Tab¶
The Overview tab provides general high level information about the study. The aim is so any users that view the record have enough information to hand. If the user has permission, these study details can be edited (Edit User Permissions).
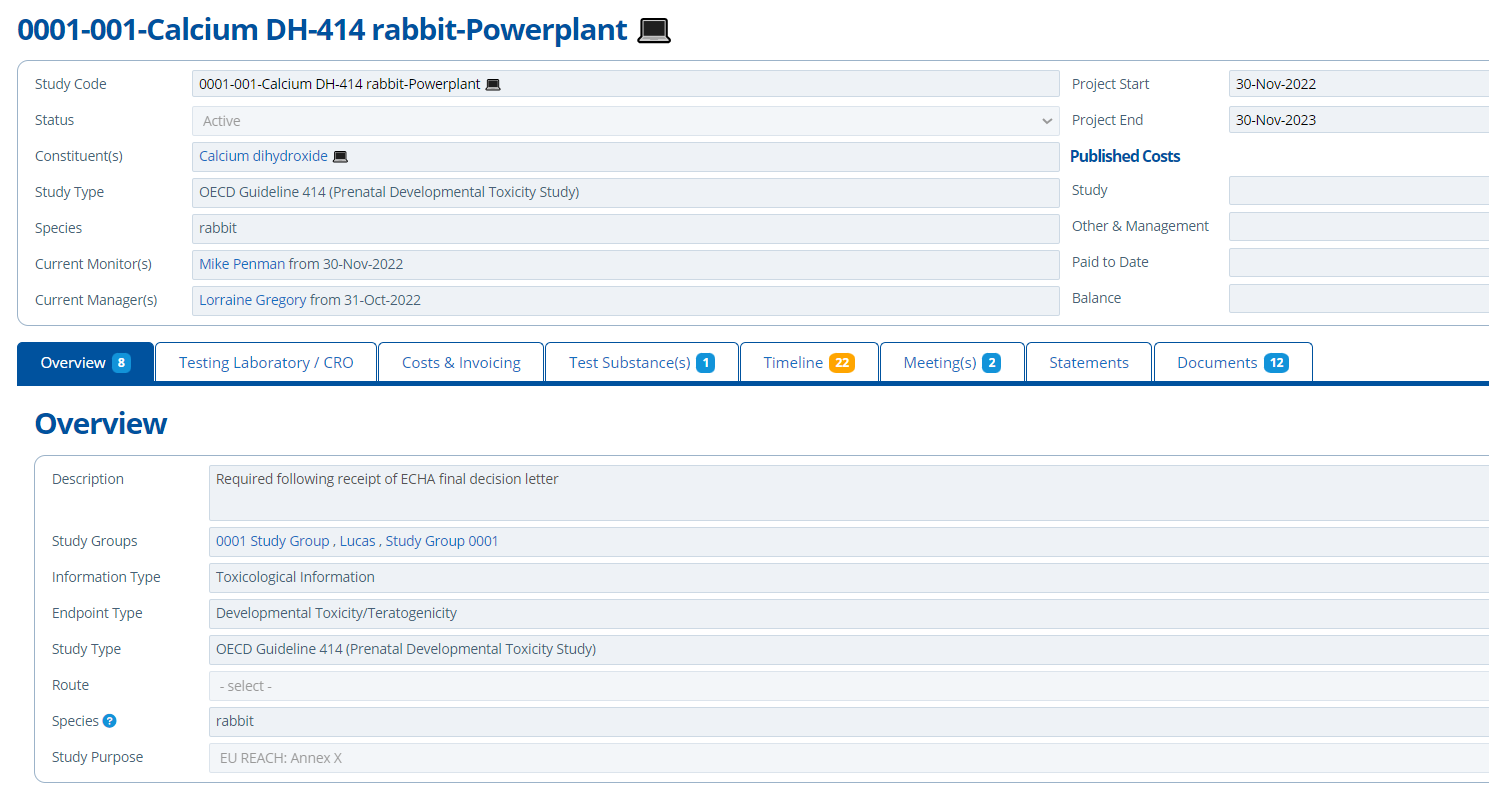
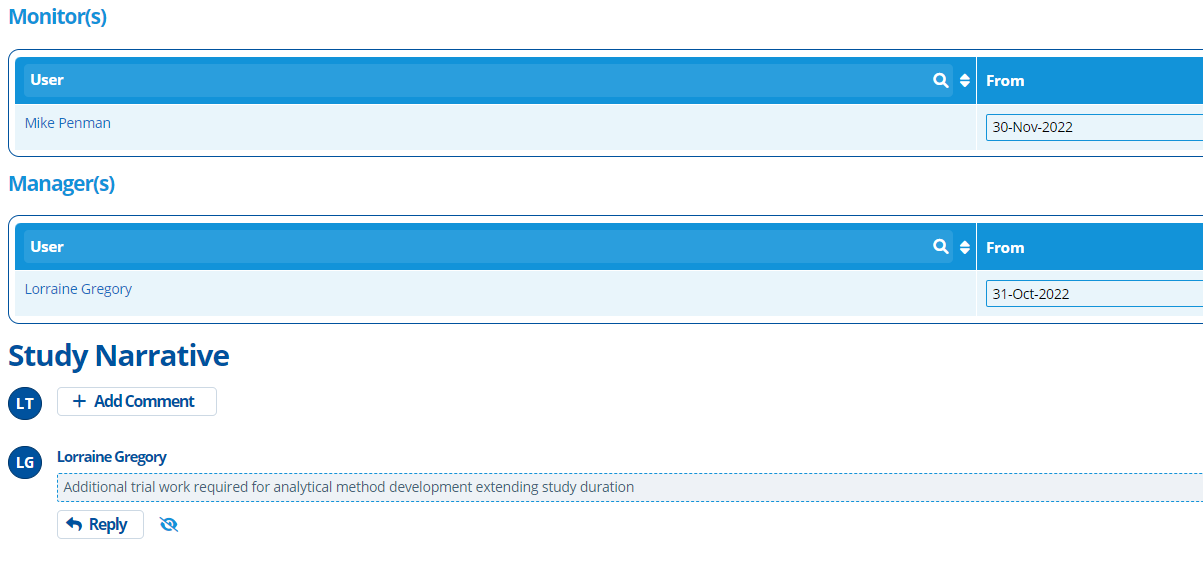
Study Monitors and Mangers can be assigned to the project. Assigning a monitor/manager involves selecting a user of the system and the date from which they are responsible. This helps with organisation and clearly indicates who is the relevant go-to person for a study. It also tracks who historically worked on it.
On the Overview there is a section for the “Study Narrative” – this is where comments / notes can be left. The comments added are usually the latest updates on the progress of a study. See Comments in Active Steward for more information.
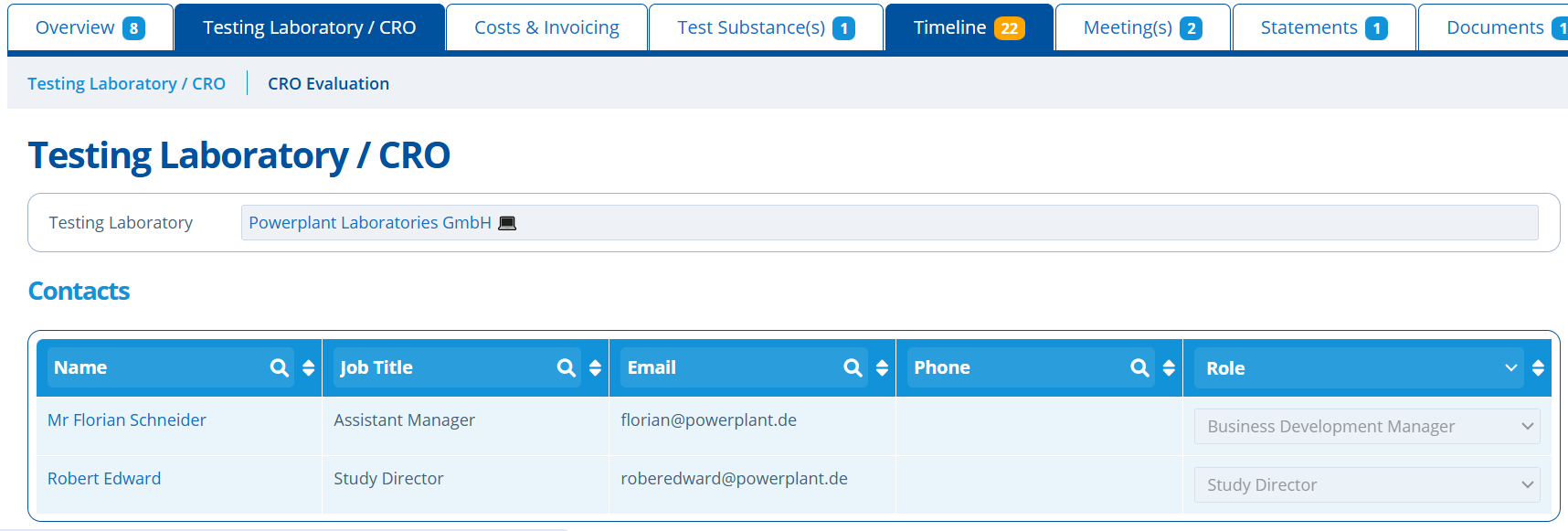
Testing Laboratory Tab¶
The Testing Laboratory / CRO tab holds information such as;
- Study Phases (called "Activities" in Active Steward).
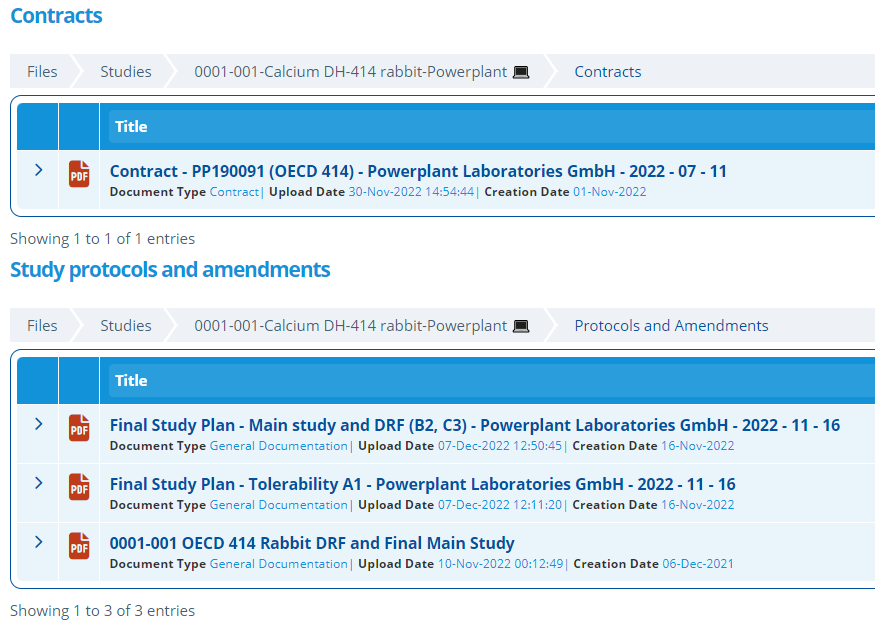
- Displays relevant Lab/CRO documentation (Contacts, Protocols and Amendments).
- Laboratory / CRO details and their specific contacts.
The CRO / lab used and the contact person(s) for this study can be selected in drop-down fields. The laboratory and contacts assigned must already exist in the system as those records will hold further useful information, some of which is pulled through and displayed on this page (Create Entity and Contacts). If the email address in the table is clicked, a new email is opened with the study code pre-populated in the subject line.
The "Activities" section on this tab is used to show the separate phases of a study. For example, the phases could be:
- Analytical Method Development and Validation
- Dose-Range Finding Developmental Toxicity Study
- Oral Developmental Toxicity Study
- Tolerability Study
For each activity created, a corresponding payment schedule will be created in the timeline tab (Create Study Activities and Set up the Timeline).
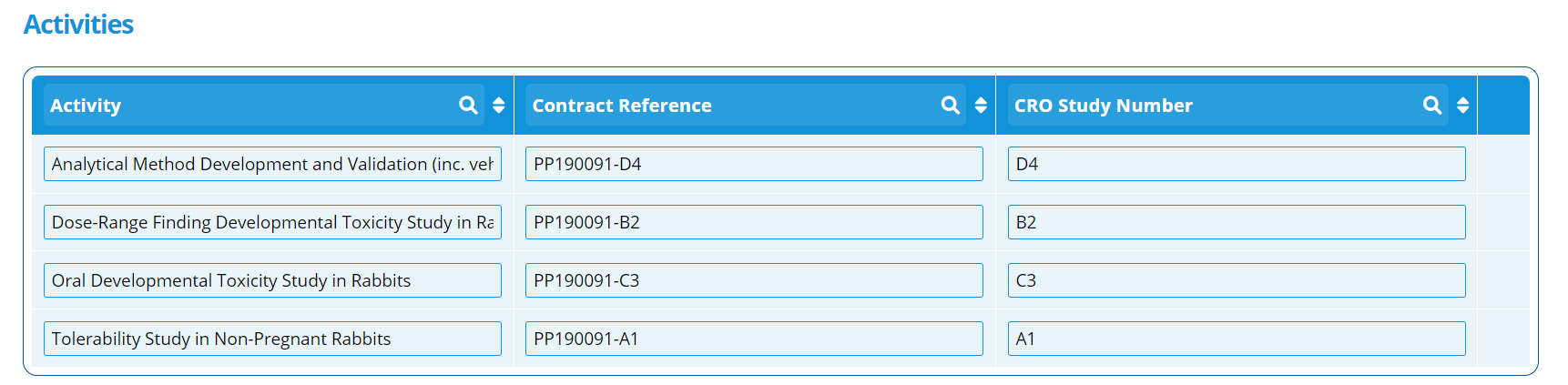
Each activity can capture the CRO Study Number and Contract Reference.
Study documents are uploaded and displayed in the section below so relevant documents can be quickly located. It is good practise to make the "Contract Reference" in the activity correspond to one of the documents names here (How to Create a Document).
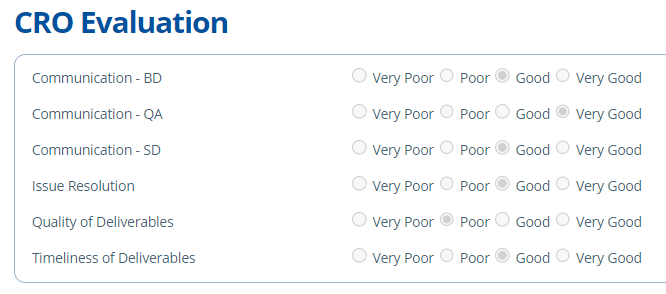
CRO Evaluation¶
This tab is used to obtain metrics and rate the working relationships with Labs/CROs. It can help determine future Lab choices and can store information for clients on how the experience was if requested.
Costs & Invoicing Tab¶
The Cost & Invoicing tab is a tool used in study financing. It includes multiple subtabs to aid invoicing and the cost sharing process. See the function of each explained below.
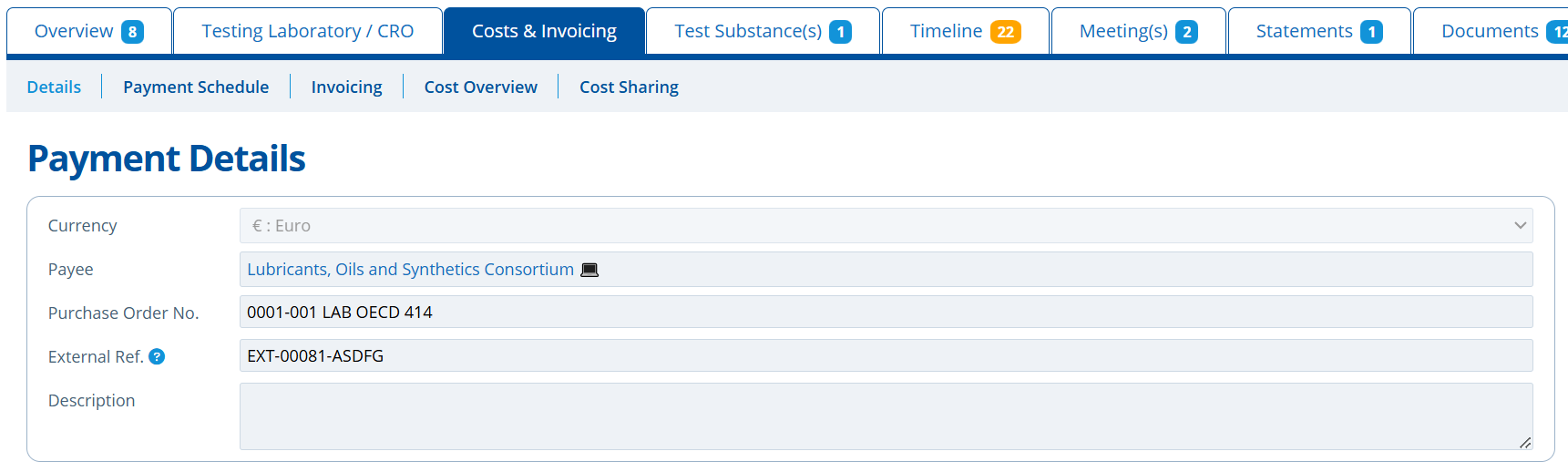
Payment Details¶
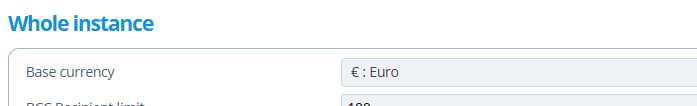
This tab stores cost information relating to the study. The Purchase Order and External Reference numbers are noted here, so it can be referred back to and used during invoice or document creation for example. The Currency field sets the currency of the Payment Schedule and study costs - by default this will be set to the "Base currency" selected in the instances configuration settings:
Payment Schedule¶
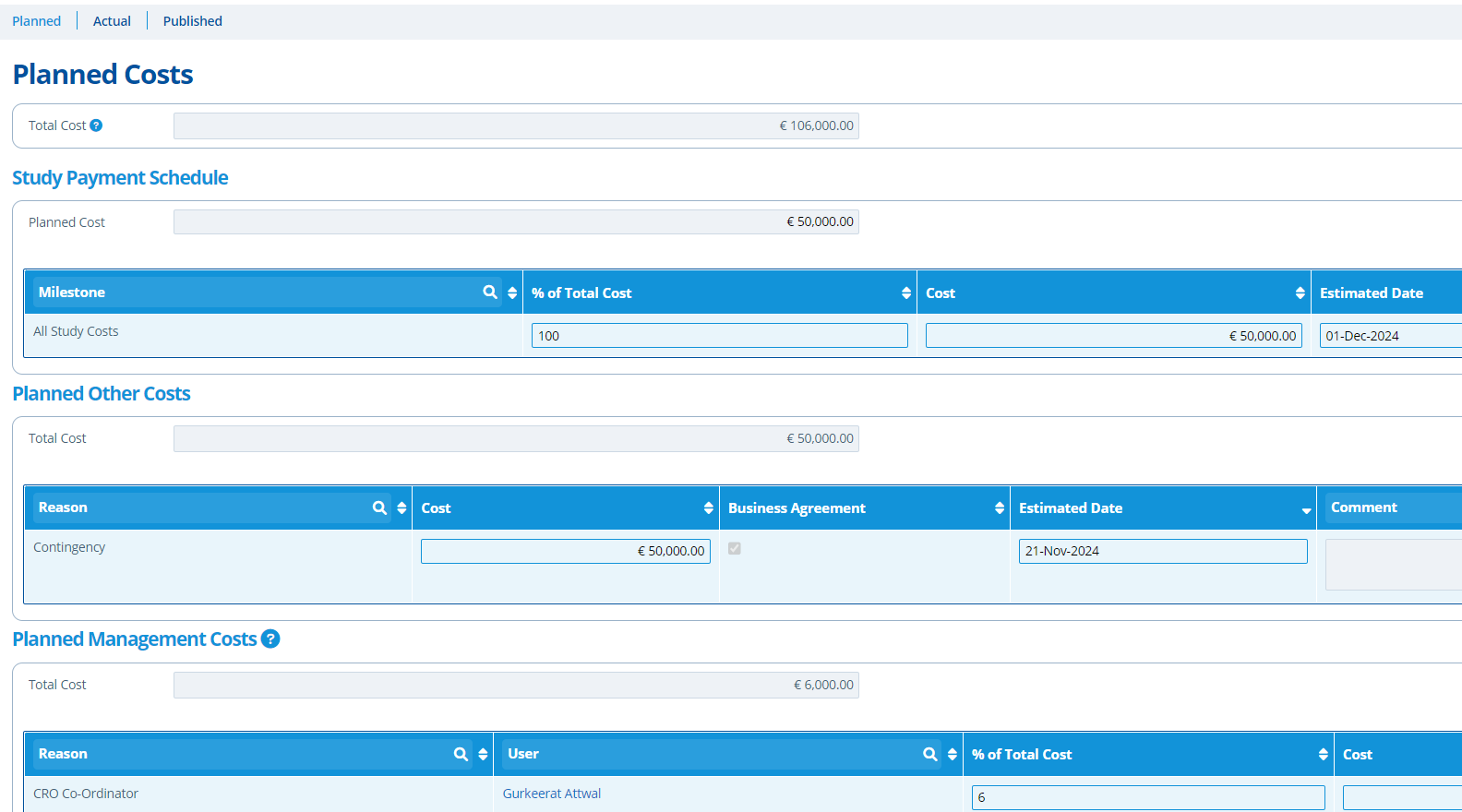
The payment schedule keeps track of planned, actual and published costs. A payment schedule outlines the costs and estimated dates of when these amounts are expected to be paid. The costs are split into types;
- Management Costs - These are the service provider (our) costs for organising, monitoring and managing a study. Management costs for all studies in the partition can be exported - see here.
- Scheduled Costs - These are the costs for the study to take place at the laboratory.
- Other Costs - This is usually contingency costs to cover any unforeseen issues. Other costs for all studies in the partition can be exported - see here.
- Planned costs are filled out before a study commences. At the planned stage the study costs are usually an estimate based on the study type and CRO/laboratory used.
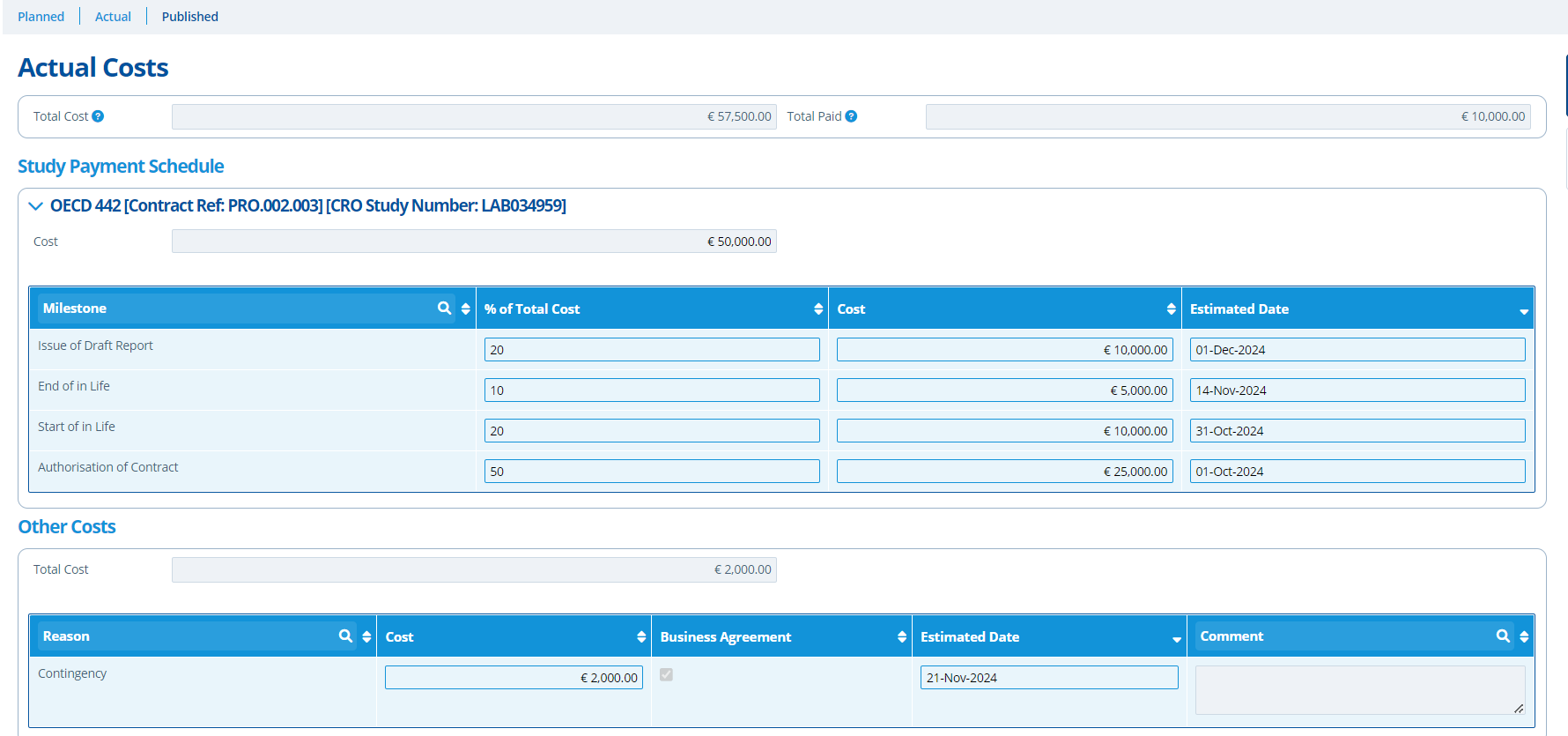
The planned costs will need updating once the study becomes active and more financial information becomes available. The updated costs are filled out on the "actual" costs section - once the study status changes to active, the planned tab cannot be edited. The payment milestones on the "Actual Costs" tab represents the cost breakdown in further detail.
The costs and payment schedule can be "Published" once it is finalised / agreed by the study manager. Published costs cascade throughout the system to be used in other areas of Active Steward such as the cost overview or campaigns (How to Create a Payment Schedule and Publish Costs).
Invoicing¶
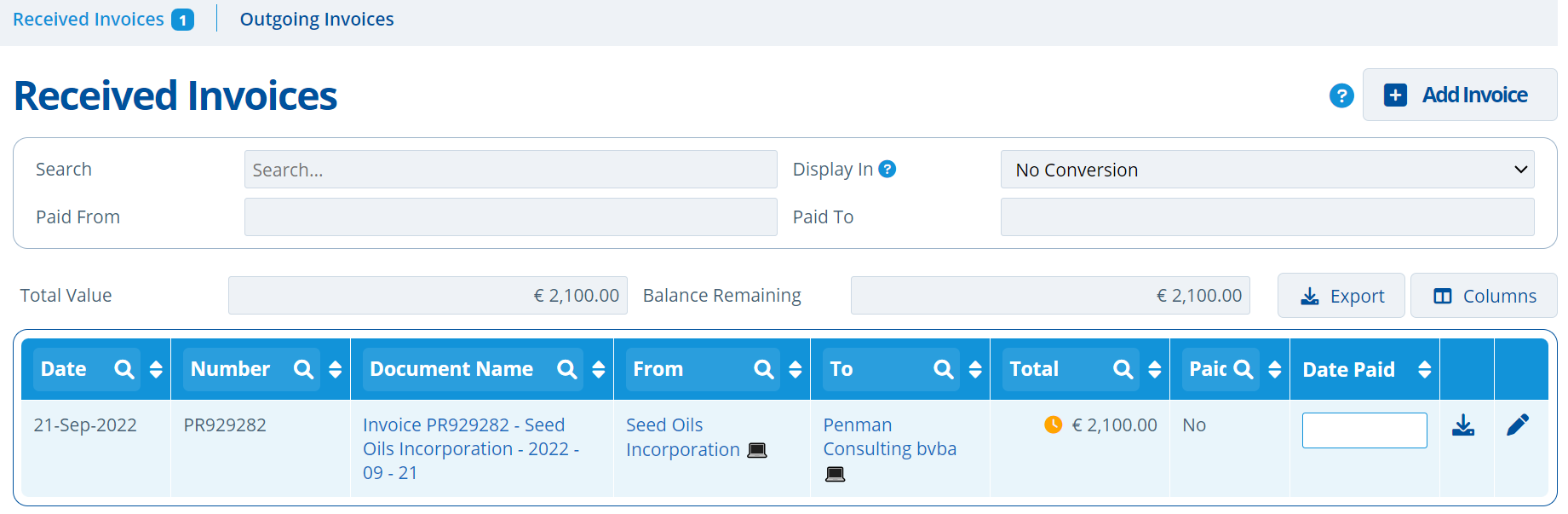
The invoicing tab displays all invoices associated with a study. It helps keep track of the current total study value and balance. The tab is split into two; "Received" invoices and "Outgoing" invoices.
- Invoice document records have fields for "To" and "From", used to tag entities that the invoice is sent to and from.
- Received invoices are usually from the CRO / laboratory for the study activity costs. Any invoice linked to the study that is not from the partitions set "Invoice 'From' Entity" will be shown on the Received tab.
- Any invoice linked to the study that is from the partitions set "Invoice 'From' Entity" will be shown on the Outgoing tab.
- The "Invoice 'From' Entity" can be chosen in the partition configuration settings.
The invoice tables help keep track of finances by;
- Marking invoice rows with a symbol when an invoice becomes overdue. The number of days for an invoice to be marked as "overdue" is set up in the configuration settings.
- Marking invoices as "Paid". This is done by populating the "Date Paid" column with the relevant date.
- Displaying the entity(ies) that the invoice is being sent from and who it is sent to.
- Displaying Total Value and Total Balance fields to give a quick overview of overall invoicing costs.
- Using the "Add Invoice" button to directly upload invoices relevant to this study.
- Searching and using filter options to locate specific invoices.
Cost Overview Tab¶
This tab provides a high level overview of a study's costs. The following information can be interpreted from the table and fields;
- Total Cost vs Total Paid
- Costs separated by Month, Quarter or Year
- How costs are shared between entities
- Costs over a specific timeline
- Costs separated by share (helpful when an entity is paying multiple shares)
- How much of the total has been invoiced out to entities so far
- Remaining balance to be paid by entities and the amount paid so far
- Amounts by type of cost (management, other or study running costs)
Invoices can be generated from information held and filtered in this overview table. Invoice generation relies on an integration - see Generate Invoices.
See the following examples of how the Cost Overview is used to draw conclusions:
Costs separated by Entity and Share:
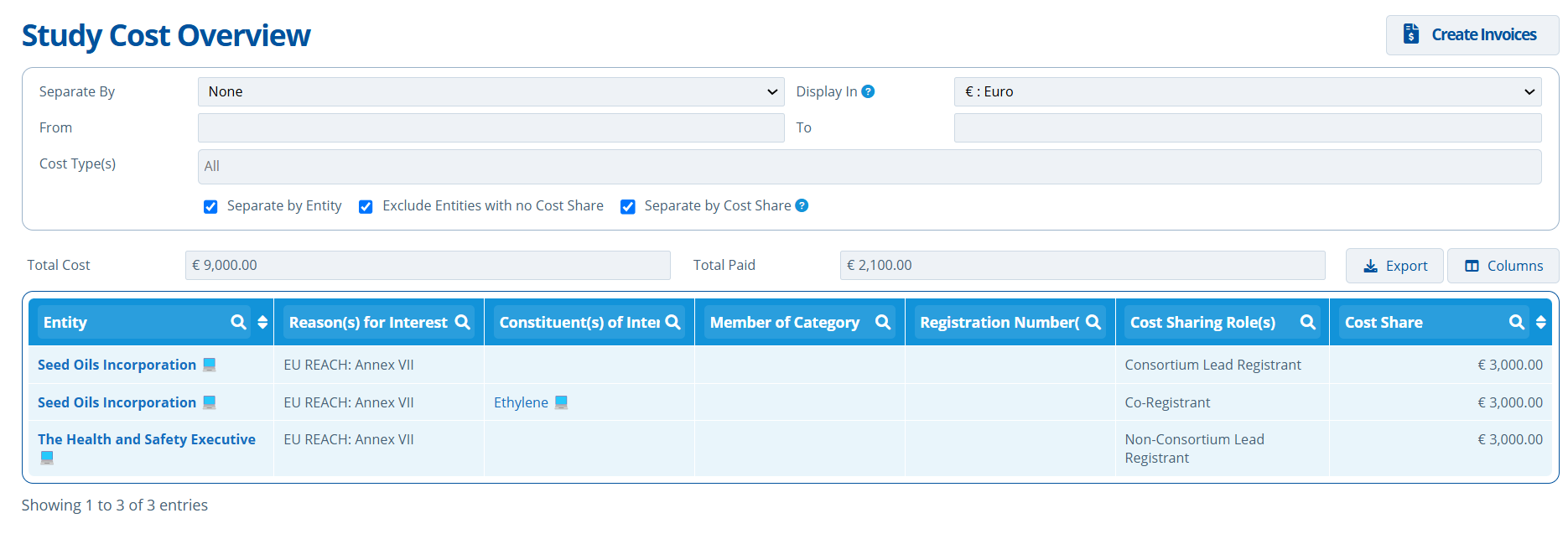
- The entity "Seed Oils Incorporation" is responsible for 2 shares totaling €6000.
- The entity "The Heath and Safety Executive" is responsible for a share of €3000.
- The Total Cost of the study is €9000, which comes from the setup payment schedule (Create Payment Schedule and Publish Costs).
- The Total Paid so far towards the study costs is €2100.
Costs separated by Year:
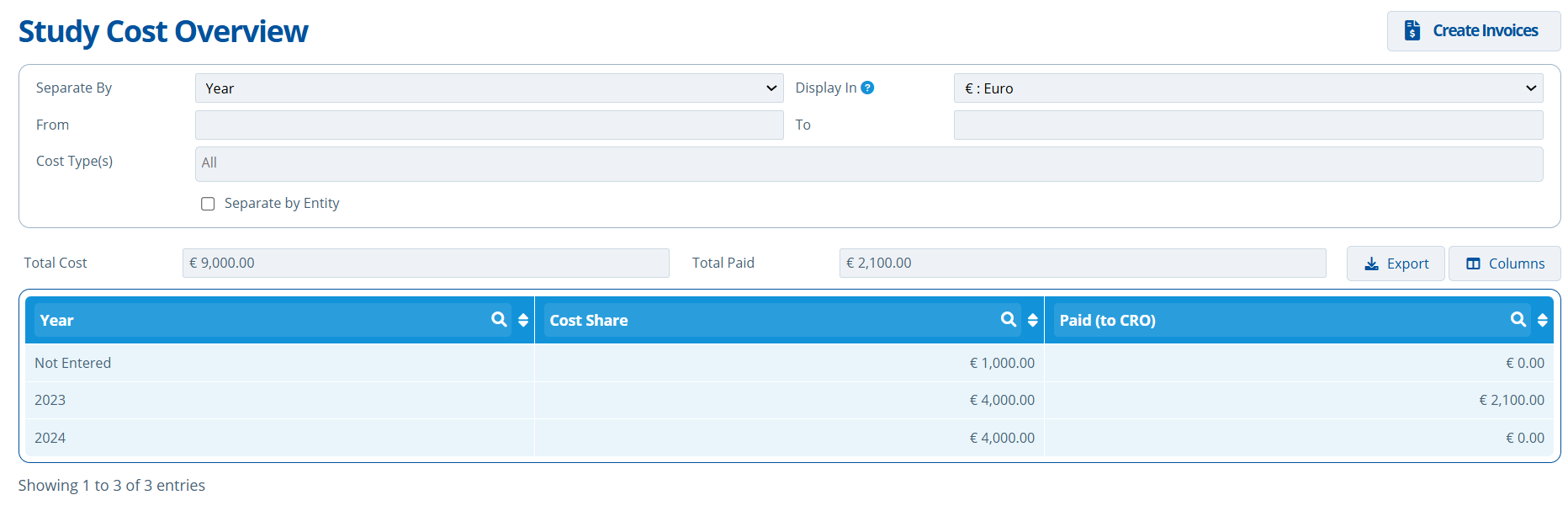
- In 2024 the total cost shared (amount due) was €4000. In 2023, €4000 was due.
- In 2023, €2100 was paid to the CRO / lab.
- "Not Entered" refers to milestones in the payment schedule with no date assigned.
Cost Sharing¶
Entities¶
The "Entities" tab is where company cost sharing is set up. Here companies (called entities in Active Steward) are linked to a study either manually, automatically or by study group. See the following tutorials for more information:
Linking entities to studies is done to set how study costs are to be shared between companies. Not all entities linked to a study will be responsible for shares - it depends on the information (cost sharing roles and reason for interest) assigned to each link. See the following example: Assign an Entity to Pay Shares Towards a Study.
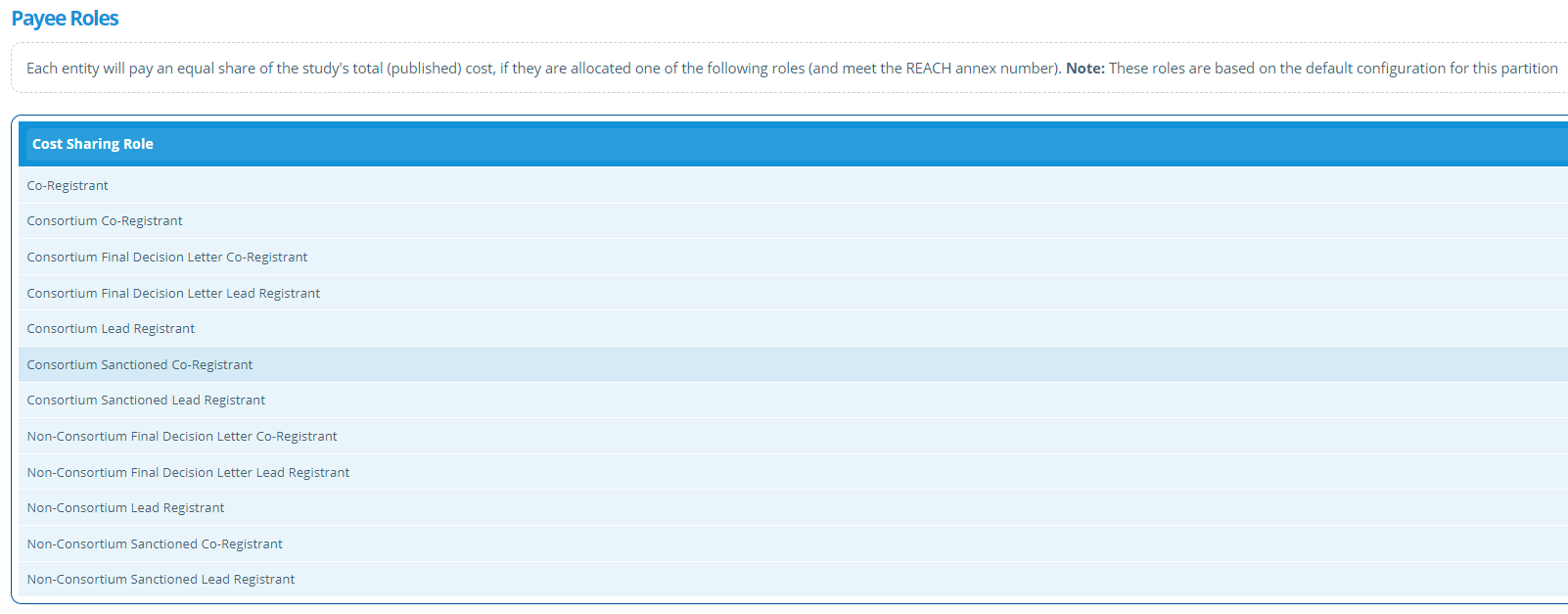
Sharing Rules¶
The sharing rules tab lists payee roles, that when given to an entity, indicates that they can pay shares towards relevant studies.
The list can be amended by editing the Study Configuration. Alternatively, the study manager can override and customise the list for a specific study by editing this tab.
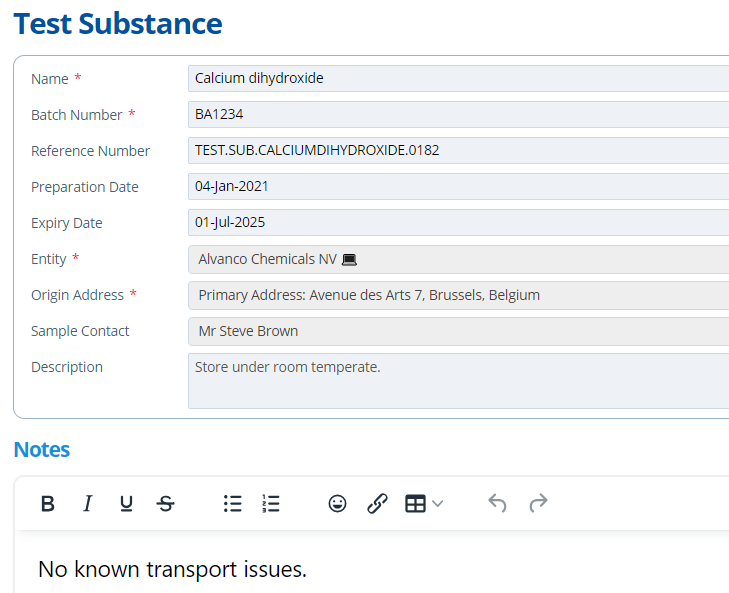
Test Substance(s) Tab¶
A study requires samples of the substance being tested on. Obtaining samples can be a logistical issue. This tab provides a space to keep track of the necessary details. Notes can be left about the samples progress and supporting documentation can be uploaded. See Add a Test Substance for more instructions.
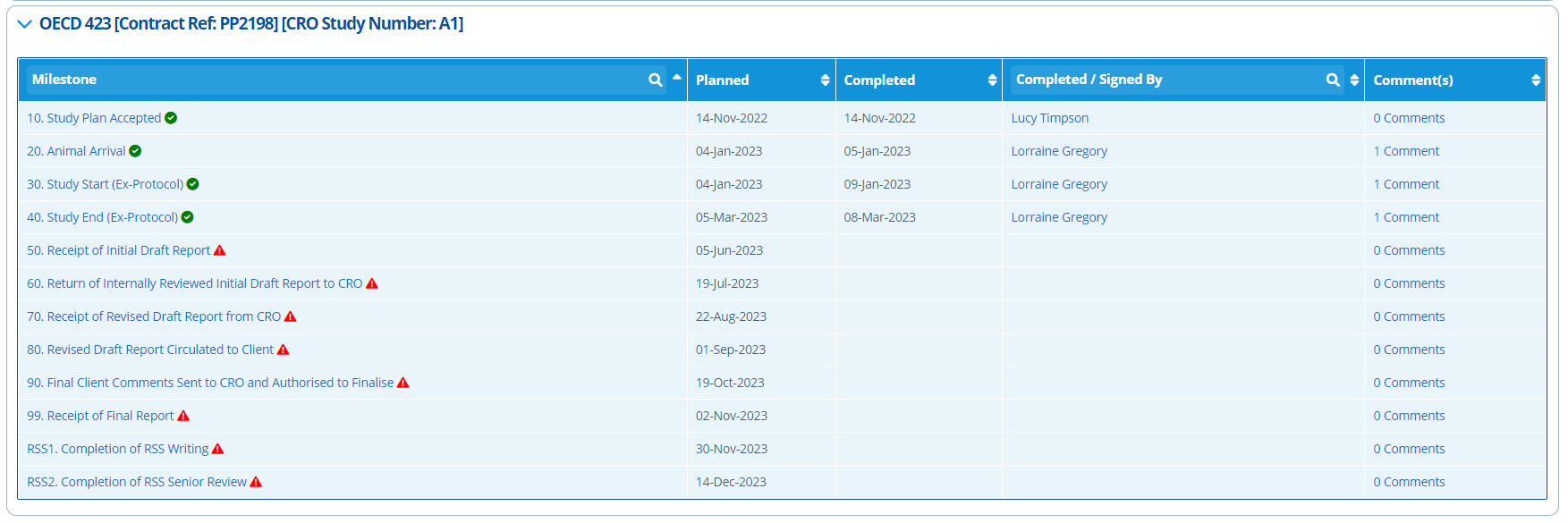
Timeline Tab¶
The Timeline tab is key for tracking project progress. Each study has a "General" timeline by default. There are also specific study timelines that can be generated - see the following tutorial for more information: Create Study Activities and Set up the Timeline.
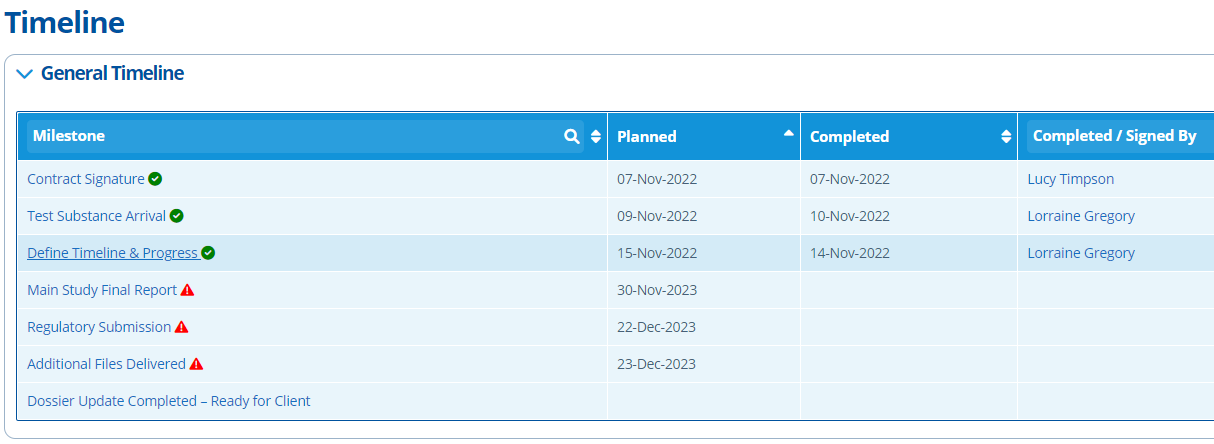
The page has "Planned" and "Completed" date fields for each activity/stage of the study. When an activity becomes overdue notifications can be sent to some users reminding them to provide a note/ update or change a planned date - see Notifications in Active Steward for more information.
Planned study dates are usually provided by Labs/CROs - the planned schedule can be put into this tab. Each activity/stage can have notes added against it, which is essential for keeping users up to date on possible delays or changes.
The study specific timeline is more detailed, whilst the general timeline is more of an overview. The timeline generated for each study activity depends on the template selected upon study creation. The timeline types to choose from are environmental, toxicology or analytical. The milestone points in study timelines can be amended by editing the study configuration.
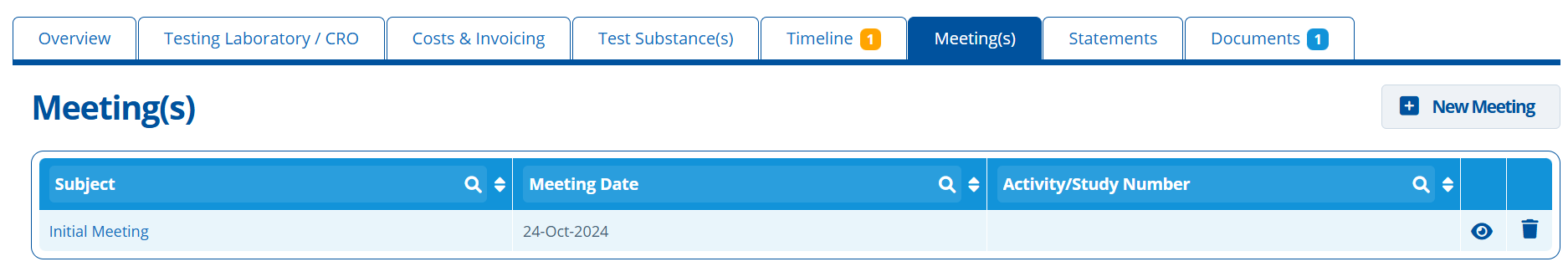
Meeting(s) Tab¶
This tab is used to generate meeting documents. The document can include attendees, dates, agenda, discussion points and actions. Using this feature means that the generated meeting documents will have similar formatting /layout each time they are created. This ensures consistency in files that are circulated to clients.
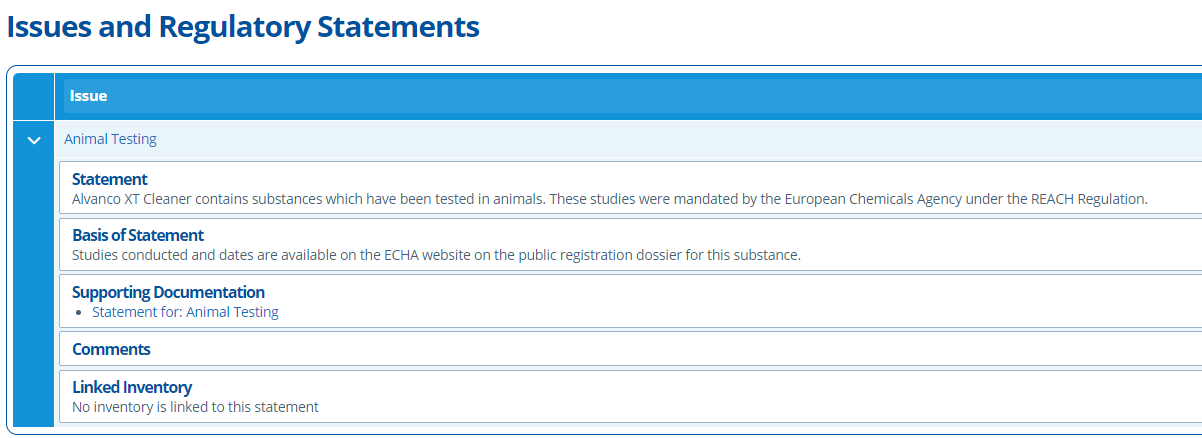
Statements Tab¶
This tab lists any Issues that are linked to the study. Study related Issues could be questions raised about testing methods, test substances or regulatory problems for example. Issues here can be used to keep track of questions raised by the client or CRO - See Create, Send and Link an Issue & Statement for more information.
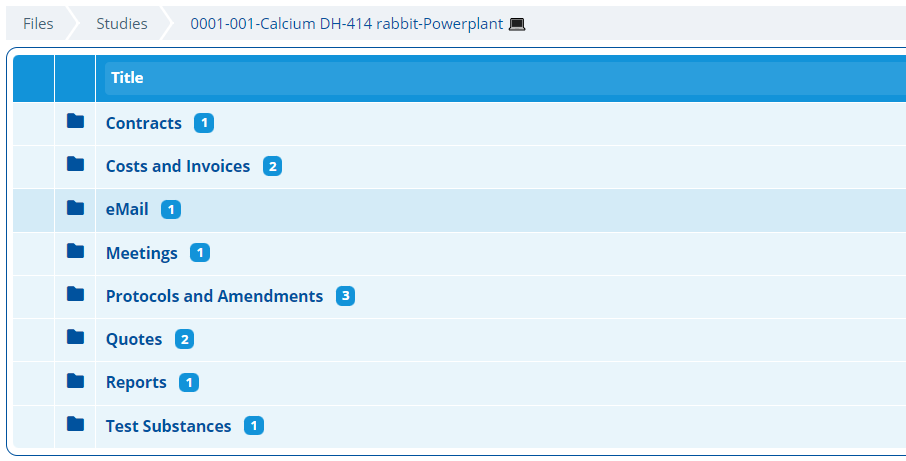
Documents¶
Each study record has a specific folder structure for storing documents. Inside the study folders there are sub folders which help organise different types of study related document, such as; Contracts, Invoices, Reports and Quotes. Documents can be automatically saved into these folders via email - Automatically Save Emails (and Attachments) to Study Folders.
Cost Overview¶
The Cost Overview screens are used to summarise costs in one place, in a table. Totals fields are available and search / filter options can be used. Information on the overviews can help draw conclusions on the financing status of various records / areas in Active Steward. See below the list of cost overview screens containing study related costs:
- Studies 👉 Cost Overview - Costs displayed are those for all studies in the partition.
- Study 👉 Costs & Invoicing 👉 Cost Overview - Costs displayed are those from this study.
- Study Group 👉 Costs 👉 Cost Overview - Costs displayed are those from studies in this study group.
- Product 👉 Studies 👉 Cost Overview - Costs displayed are those from studies that are linked to this product.
- Constituent 👉 Studies 👉 Cost Overview - Costs displayed are those from studies that are linked to this constituent.
- Category Group 👉 Studies 👉 Cost Overview - Costs displayed are those from studies that are linked to this category group. The overview also has the option to show studies linked to the category groups constituents.
- Entity 👉 Studies 👉 Cost Overview - Costs displayed are those from studies, where the entity is assigned to pay shares.
- Entity Group 👉 Studies 👉 Cost Overview - Costs displayed are those from studies, where an entity from this entity group is linked to the study.
Study costs come from study payment schedules.
The overviews have many options and filters to use, such as;
- Choose the type of cost to show; Management, Other or Scheduled Study Costs.
- Select a currency to display costs in - by default this is the "Base currency" selected in the instance configuration settings.
- The option to separate rows by Year, Month or Quarter*.
- Choose a custom timeframe to view the costs over. Costs included are milestones from payment schedules that have an estimated date within the selected "From" and "To" date. It also includes management costs during that timeframe (the management costs are split evenly by month over the whole study duration).
- Use the table headers to search - for example, search for a specific entity to only view their costs.
Invoices can be generated from any of the cost overview screens. The invoices generated are dependent on the overview used and the filter options applied to it.
See the following examples on how to interpret the Cost Overview:
Separate By Year and Entity:
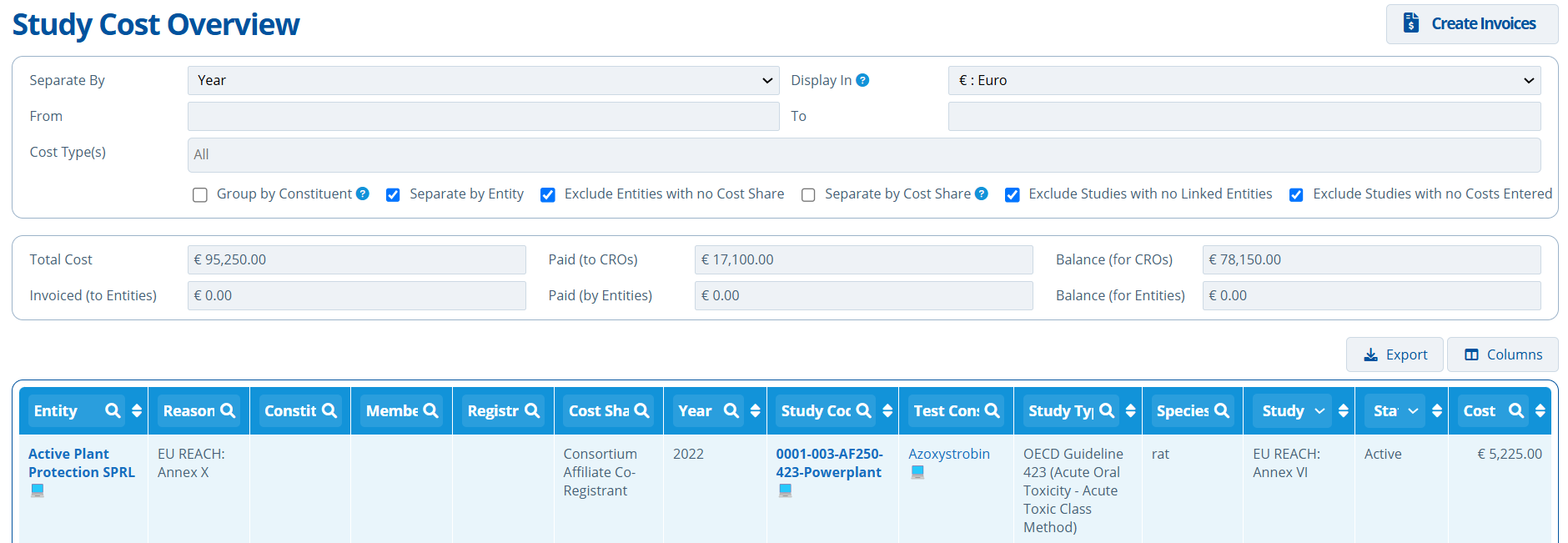
- The entity "Active Plant Protection SPRL 💻" is responsible for paying a total of €5225.50 in the year 2022 towards the study "0001-003-AF250-423-Powerplant 💻".
Separate By Constituent:
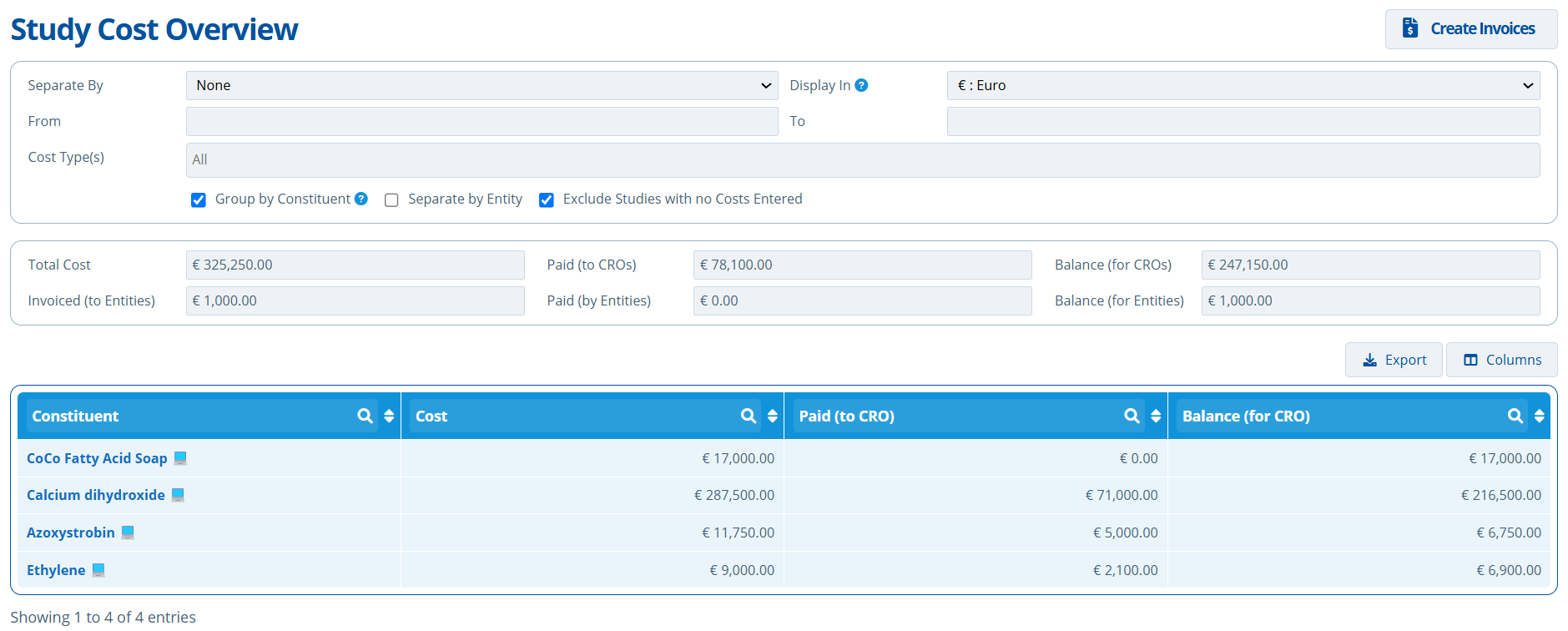
- The total study costs for the constituent Azoxystrobin is €11,750. €5000 has been paid so far. The total study costs for all constituents in the partition is €325,250.
Study Groups¶
Study Groups are usually used when substances are assessed as a group. This is useful because multiple studies are able to "inherit" and use the same information from the group. Using study groups help cut down on admin time and makes it clear what studies are being run together - See Create a Study Group.